Scroll to:
Development of approaches for evaluating the pharmacokinetics of meropenem during endolymphatic antibiotic treatment in critically ill patients
https://doi.org/10.47093/3034-4700.2025.2.2.24-35
Abstract
Введение: Использование традиционных методов введения лекарств при антибактериальной терапии пациентов в критическом состоянии может оказаться недостаточным, поскольку минимальная ингибирующая концентрация, необходимая для эффективной терапии, может не поддерживаться необходимое количество времени из-за особенностей фармакокинетики пациентов. В качестве альтернативного подхода предложена эндолимфатическая терапия.
Цель: Оценка фармакокинетики меропенема при эндолимфатической антибактериальной терапии и ее сравнение с внутривенным путем введения.
Материалы и методы: Образцы крови пациентов, получавших меропенем эндолимфатически (n = 1) и внутривенно (n = 1), анализировали с помощью высокоэффективной жидкостной хроматографии с диодно-матричным детектированием и высокоэффективной жидкостной хроматографии с тандемной масс-спектрометрией с ионизацией электрораспылением.
Результаты: При внутривенном и эндолимфатическом введении меропенема минимальная концентрация в плазме в равновесном состоянии составила 10 мкг/мл и 16,39 мкг/мл, максимальная концентрация в плазме в равновесном состоянии – 42,41 мкг/мл и 42,57 мкг/мл, площадь под кривой в равновесном состоянии – 363,997 мкг·ч·мл-1 и 521,86 мкг·ч·мл-1, среднее время удержания – 8,446 и 11,365 часов.
Заключение: Наши результаты демонстрируют более длительную персистенцию меропенема в кровотоке после эндолимфатического введения, что указывает на предпочтительную фармакокинетику. Кроме того, минимальная концентрация в плазме в равновесном состоянии после эндолимфатического лечения оставалась на высоком уровне, превышая минимальную ингибирующую концентрацию. Однако для получения надежных подтверждений преимуществ эндолимфатического пути введения необходимы дальнейшие исследования на более крупных когортах.
Keywords
For citations:
Turenko V.N., Ramenskaya G.V., Karimova Z.К., Esipov А.V., Pavlov А.I., Filippov А.V., Kislenko А.М., Kharitonov V.V., Yusuf E., Korobov S.S., Оrekhov S.N., Smirnov V.V. Development of approaches for evaluating the pharmacokinetics of meropenem during endolymphatic antibiotic treatment in critically ill patients. The BRICS Health Journal. 2025;2(2):24-35. https://doi.org/10.47093/3034-4700.2025.2.2.24-35
Введение
Карбапенемы относятся к классу β-лактамных антибиотиков широкого спектра действия, структурно близких к пенициллину, где атом серы замещен на атом углерода [1]. При прочих равных условиях среди всех карбапенемов в настоящее время предпочтение отдается меропенему из-за большей активности в отношении грамотрицательных бактерий, которая усиливается возможностью применения более высоких доз вследствие меньшей нейротоксичности [2]. Меропенем активно применяется в современной клинической практике в Российской Федерации и ряде других стран. В Российской Федерации он включен в ряд действующих клинических рекомендаций Министерства здравоохранения Российской Федерации, например, как препарат выбора для пациентов с тяжелой внебольничной пневмонией и факторами риска инфицирования P. aeruginosa и энтеробактериями 1,2,3 .
The most important pharmacokinetic/pharmacodynamic parameter of carbapenems is the ratio of the time when the concentration of the free drug exceeds the minimum inhibitory concentration (MIC) to the time between injections of the drug. This is expressed as a percentage time above MIC and requires 20% to achieve bacteriostatic effect of carbapenems and 40% to achieve maximum bactericidal effect. The MIC value depends on a specific microorganism and ranges from 0.03 to 8 µg/ml, reaching in some cases up to 32 µg/ml for resistant pathogens. It is worth noting that the value of this indicator required to achieve a certain effect is different for different groups of beta-lactams [3][4]. Patients with severe infectious diseases are at risk of reaching only subtherapeutic concentrations (insufficient percentage time above MIC) due to pathophysiological changes affecting the pharmacokinetics of the drug, for example, renal and hepatic dysfunction, increased renal clearance, increased apparent volume of distribution [5]. Plasma concentrations of carbapenems may be insufficient with recommended dosages for severely ill patients with increased volume of distribution or for dialysis patients [2].
The use of intravenous or intramuscular routes of administration in antibiotic therapy of severe infectious diseases is not enough to achieve the necessary therapeutic effectiveness and eliminate pathogens. As an alternative approach, the use of endolymphatic therapy has shown its effectiveness in military field surgery, oncology, urology, gynecology, traumatology, phthisiology, as well as in the treatment of acute surgical diseases of the abdominal cavity [6]. Endolymphatic administration allows you to increase the flow of the drug into the pathological site (inflammation, wound, degenerative tissues, etc.) due to transport mediated by immunocompetent cells [7]. With endolymphatic administration, antibiotics are delayed in the lymphatic system for up to 24–48 hours, creating a depot of the drug in the body, followed by a slow dosage of them into the blood. When in lymph, it is expected that up to 50% of the dose of the administered antibiotic is adsorbed on the surface of lymphocytes, in addition, unstable coupling of drugs with immunoglobulins occurs, which ensures the entry of antibiotics into the site of inflammation together with immunocompetent cells and immunoglobulins [8]. Endolymphatic administration involves direct administration of the drug into the lymph in the following ways: catheterization of the peripheral lymphatic vessel (antegrade method) or superficial lymph node (intranodular method), through the thoracic lymphatic duct (retrograde method). A number of authors note the advantage of endolymphatic methods of administering drugs compared to traditional ones: faster recovery and recovery of patients, shorter length of hospital stay, reduced number of complications, reduced side effects of drugs [9].
Since meropenem refers to time-dependent antibiotics, that is, the effectiveness of treatment depends on the time of retention of the antibiotic concentration in the site of infection above the MIC for this pathogen, an important factor ensuring the effectiveness of therapy is therapeutic monitoring of the antibiotic concentration in order to adjust the dose in case of insufficiency of the percentage time above MIC indicator, which requires a sensitive, specific and suitable for routine analysis method for the quantitative determination of antibiotic in biosamples. The high-performance liquid chromatography (HPLC) with diode-array detection (HPLC-DAD) method was developed and validated to monitor the concentration of meropenem in blood plasma, and HPLC with electrospray ionization tandem mass-spectrometry (HPLC-ESI-MS/MS) to assess the concentration in white blood cells. The aim of this study was the evaluation of meropenem pharmacokinetics during endolymphatic antibiotic therapy and its comparison to intravenous administration route using HPLC-DAD and HPLC-ESI-MS/MS methods.
Materials and methods
Selection of biosamples
Two critically ill patients aged 36 (patient 1) and 45 (patient 2) with sepsis, hospitalized in April 2023, received 1 g of meropenem twice a day participated in the study, the first received meropenem intravenously, the second endolymphatically. Blood plasma was selected as possible biosamples for analysis to study the pharmacokinetics of meropenem during endolymphatic administration and further therapeutic monitoring. Based on the literature data on meropenem plasma concentration levels, HPLC-DAD was selected as the assay method.
Also, to evaluate the hypothesis of the delivery of antibiotics by immunocompetent cells to the site of inflammation, it was proposed to analyze lysates of leukocytes previously isolated from whole blood. Given the lower expected concentrations than plasma, HPLC-ESI-MS/MS was selected as the assay method.
Blood sample preparation
To obtain plasma, whole blood was centrifuged at 2500 revolutions per minute (RPM) for 15 minutes, and the supernatant was transferred to an individual tube.
300 µL of plasma was transferred to a 1.5 mL microtube, 600 µL of acetonitrile was added, and centrifuged at 13000 RPM for 10 minutes to precipitate proteins. The supernatant was transferred to an individual vial and the samples were stored at -70 °C until analysis.
Sample preparation of peripheral blood mononuclear cells
To obtain leukocyte lysates, the peripheral blood mononuclear cells (PBMCs) isolation method followed by lysis was used. A whole blood sample (5 mL) was transferred to saline tube 1 (5 mL). Ficoll solution (3 mL) was then added to tube 2 (15 mL). Ficoll solution has a specific density of 1.077 g/ml, which ensures the correct separation of blood layers. After neatly layering the blood (from tube 1) onto the gradient (tube 2), centrifugation was performed at 400 g (20 min). The principle of the gradient separation method is based on the separation of blood cell elements by size when centrifuged in a Ficoll gradient with a specific density of 1.077 g/ml.
After centrifugation, various layers of whole blood were visible. The main cellular component of blood is red blood cells, which make up 45% of the total blood volume. The remaining 55% is plasma. The PBMCs fraction lies just below the plasma layer. It includes lymphocytes (T- and B-cells), naive cells, monocytes, dendritic cells, stem cells. Granulocytes, which are heavier than PBMCs, are located between their layers and the erythrocyte layer.
Next, 3 mL of the formed PBMCs ring was sampled over the entire cross-sectional area of the tube by pipetting through the plasma fraction. The PBMCs fraction was transferred to another tube with 10 mL saline. The contents of the tube were centrifuged at 400 g (7 min). As a result, PBMCs settled at the bottom of the tube, 500 µl of guanidine lysis solution was added to PBMCs to disrupt the leukocyte membrane. The last stage of sample preparation is protein precipitation by adding 400 µl of acetonitrile to 200 µl of PBMCs lysate and centrifuged at 13000 RPM for 10 minutes. The supernatant was transferred to an individual vial and the samples were stored at -70 °C until analysis.
High-performance liquid chromatography with diode-array detection development
A system consisting of an Agilent Infinity II 1260 high-performance liquid chromatograph equipped with a gradient pump, column thermostat, degasser, manual input and diode-array detector was used for chromatographic separation and detection of meropenem in plasma samples.
The following reagents and materials were used: acetonitrile (HPLC grade ≥99.9%), ammonium acetate (≥98%), acetic acid (≥99%), concentrated ammonia solution, purified water (Milli-Q) for HPLC, meropenem trihydrate drug substances (series: 110522; manufacturer: Sintez OJSC), intact plasma samples.
To prepare mobile phase (MP) A, 1.925 g of ammonium acetate was placed in a 1000 mL resistant glass bottle and dissolved in 1000 mL of water, 1.925 mL of acetic acid was added, mixed, and the pH of the solution was adjusted to 6.0 ± 0.1 with concentrated ammonia solution. The resulting solution was filtered through a 0.45 µm membrane filter and degassed. To prepare MP B, 1000 mL of acetonitrile was placed in a 1000 mL refractory glass bottle.
To validate the method and further quantify, model reference standards prepared by mixing 500 µL of the corresponding working solution with 500 µL of intact plasma were used, after which sample preparation was carried out as described above. The concentrations of the obtained standard calibration solutions corresponded to the following concentration range: 5, 10, 25, 50, 100, 250, 500, 1000 µg/mL.
Development of high-performance liquid chromatography with electrospray ionization tandem mass-spectrometry procedure
A system consisting of an Agilent Infinity II 1290 high-performance liquid chromatograph equipped with a gradient pump, column thermostat, thermostatic multisampler, degasser and ESI-MS/MS detector was used for chromatographic separation and detection of meropenem in leukocyte lysates.
The following reagents and materials were used: acetonitrile (HPLC grade ≥99.9%), formic acid (≥99%), purified water (Milli-Q) for HPLC, meropenem trihydrate drug substances (batch: 110522; manufacturer: Sintez OJSC), intact plasma samples.
To prepare MP A, 1000 mL of water and 1000 mL of formic acid were added and mixed in a 1.0 mL refractory glass bottle. To prepare MP B, 1000 mL of acetonitrile was placed in a 1000 mL resistant glass bottle and 1.0 mL of formic acid was added and mixed.
To validate the method and further quantify, model reference standards prepared by mixing 500 µL of the corresponding working solution with 500 µL of intact plasma were used, after which sample preparation was carried out as described above. The concentrations of the obtained standard calibration solutions corresponded to the following concentration range: 0.01, 0.05, 0.25, 0.5, 1, 2.5, 5, 7.5 and 10 µg/mL.
Validation of bioanalytical methods
The methods were validated in accordance with the rules for conducting bioequivalence studies of drugs within the Eurasian Economic Union (approved by decision No. 85 of the Council of the Eurasian Economic Commission of 03.11.2016) for the following validation parameters: specificity, limit of quantification, linearity, analytical range, precision (repeatability), accuracy, transfer effect, matrix effect, stability. All obtained values complied with the regulatory documentation requirements.
Results
High-performance liquid chromatography with diode-array detection
To develop the HPLC-DAD method, a chromatographic column was selected: Agilent Eclipse Plus C8, 4.5 × 150 mm in size, octasilane (C8) sorbent, 5 microns. An Eclipse Plus C18 2.1 × 12.5 mm, 5 µm protective column was used to protect the main column from biological sample related substances. The MP composition was optimized as follows: MP A 25 Mm ammonium acetate buffer pH 6.0 ± 0.1, MP B acetonitrile in a ratio of 89:11, the elution mode is isocratic. Oven temperature 30 °C, injection volume 20 µL. To select the wavelength of detection, the ultraviolet spectrum of the meropenem substance solution was recorded (Fig. 1), the obtained maximum absorption corresponded to the literature data and amounted to 298 nm. The retention time of the meropenem peak was about 3 minutes.
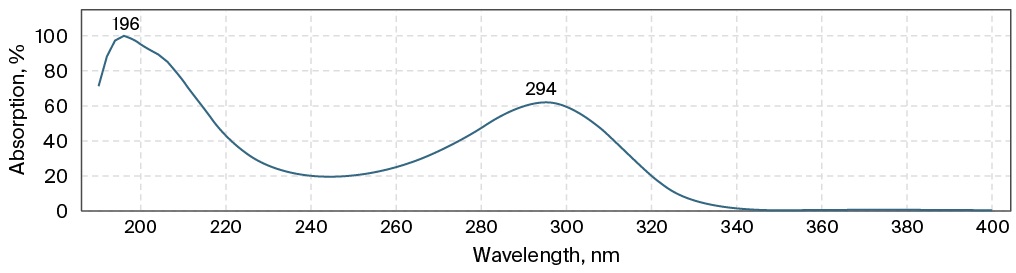
FIG. 1. The ultraviolet spectrum of the meropenem substance solution
Typical chromatograms of the blank, the lowest concentration standard calibration solution (5 µg/mL) and the patient’s plasma sample with maximum concentration are presented in Fig. 2. The developed method was used to analyze the blood plasma of two seriously ill patients receiving 1 g of meropenem twice a day, at 9 am and 5 pm intravenously and endolymphaticaly, respectively. Blood was collected at 0, 0.083, 1, 2, 3, 4, 5, 6, 8, 12, 24, 27 and 28 hours. Comparative pharmacokinetic parameters (Table) were calculated from the concentrations found.
Table. Comparative pharmacokinetic parameters for intravenous and endolymphatic routes of administration
Route of administration | Minimum plasma concentration at steady state, µg/ml | Maximum plasma concentration at steady state, µg/ml | Area under the curve at steady state, µg·h·ml-1 | Mean residence time, h |
Intravenous | 10 | 42.41 | 363.997 | 8.446 |
Endolymphatic | 16.39 | 42.57 | 521.86 | 11.365 |
A 
B 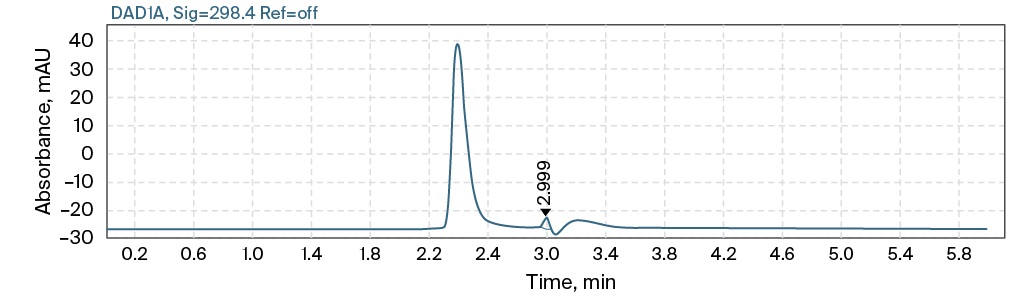
C 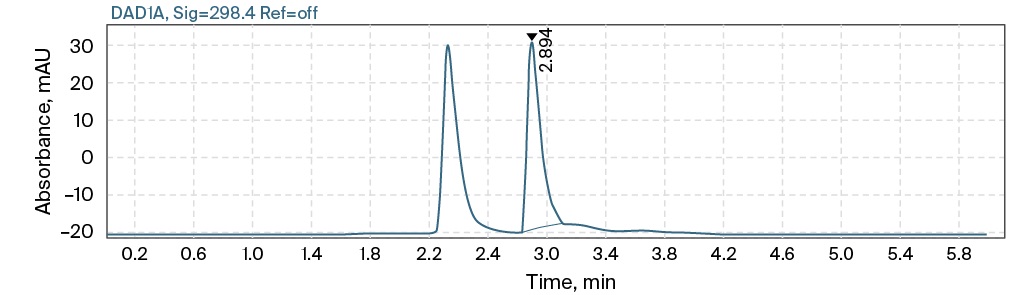
FIG. 2. Typical high-performance liquid chromatography with diode-array detection chromatograms of blank (A), lowest concentration standard calibration solution (B), patient’s plasma sample with maximum concentration (C)
From the obtained pharmacokinetic parameters, it can be seen that, despite comparable maximum plasma concentration at steady state with intravenous and endolymphatic routes of administration, in the second case, the drug remains in the body for a longer time, which demonstrates the mean residence time indicator. Also, with endolymphatic administration, minimum plasma concentration at steady state remains at a fairly high level, exceeding MIC = 12 µg/ml, which is critical for achieving a therapeutic effect during antibiotic therapy.
High performance liquid chromatography with electrospray ionization tandem mass-spectrometry
The HPLC-ESI-MS/MS method was developed on a chromatographic column for ultra HPLC “Zorbax Eclipse C18”, C18, 50 × 2.1 mm, 1.7 µm. An Eclipse Plus C18 2.1 × 12.5 mm, 5 µm protective column was used to protect the main column from biological sample related substances. The MP composition was selected as follows to obtain a positively charged parent ion of meropenem: MP A 0.1% formic acid in purified water, MP B 0.1% formic acid in acetonitrile, elution mode isocratic, solvent ratio 95:5%. Oven temperature 30 °С, injection volume 5 µL. To select the multiple reaction monitoring transition for meropenem detection, the mass spectrum was recorded and the most intense product ion 68.1 was selected (Fig. 3). ESI-MS/MS parameters were optimized to achieve the highest sensitivity of the method: precursor ion 384.2; product ion 68,1; gas temp 350 °С; gas flow 9 l/min; sheath gas flow 9 l/min; ion spray voltage 5500; collision energy 51.
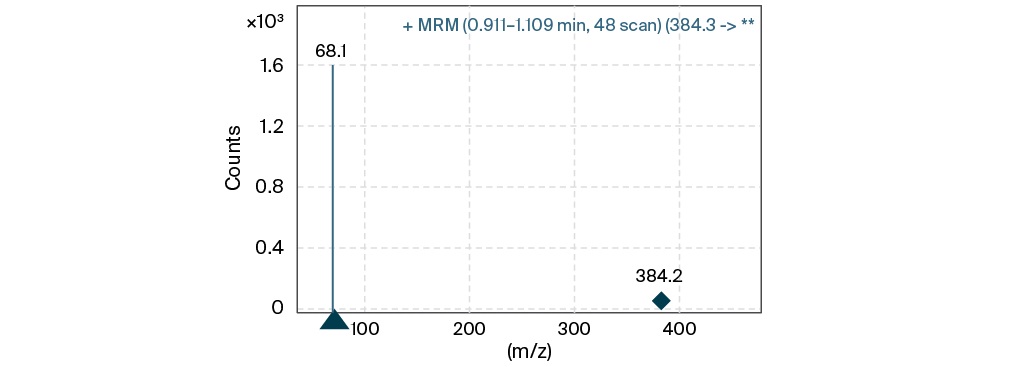
FIG. 3. Multiple reaction monitoring transition of meropenem
The retention time of meropenem was approximately one minute, typical chromatograms of blank, blood plasma and leukocyte lysate are shown in Fig. 4. These data demonstrate the concentrations of meropenem in leukocyte lysates at intravenous administration route at the limit of determination level. In the endolymphatic route of administration, concentrations of meropenem in lysate ranged from low limit of quantification to 30 ng/mL, which is associated with a high antibiotic content in the lymphatic system when directly injected into lymph.
A 
B 
C 
FIG. 4. Typical high performance liquid chromatography with electrospray ionization tandem mass-spectrometry chromatograms of blank (A), blood plasma (B) and leukocyte lysate (C)
Discussion
Meropenem demonstrates significant pharmacokinetic variability across different patient populations, underscoring the need for individualized dosing strategies. To achieve the necessary therapeutic effect, various dosage regimens can be considered, including changing the duration of the infusion and the dose administered as well as the use of alternative routes of administration [10][11]. Despite the lower bioavailability of meropenem for routes of administration other than intravenously, the time when the concentration of the free drug exceeds MIC increases [12][13]. Considering the data obtained on the steady-state concentrations in this study, this information confirms the above statement – minimum plasma concentration at steady state for intravenous route of administration was 10 µg/ml, for endolymphatic was 16.39 µg/ml, which leads to an increase in the time when the concentration of the free drug exceeds required MIC. It is necessary to optimize the dosage regimen to achieve the required MIC for patients with pathophysiological changes, for example, kidney function or in patients with severe burns, amputated limbs, because these changes affect the pharmacokinetics of the antibiotic [14–16]. The use of therapeutic drug monitoring is an effective approach to optimize dosing, ensuring improved therapeutic outcomes while reducing the risk of toxicity and reducing the development of antibiotic-resistant bacteria [17][18].
A study of the inflammatory exudate of patients receiving meropenem intravenously shows high concentrations of meropenem almost comparable to plasma concentrations [19]. Administration of the antibiotic directly into the lymphatic system will theoretically increase the concentration of meropenem in exudate, what can be mediated by the delivery of meropenem to the inflammatory site by immunocompetent cells, which requires further research. According to our data, the concentrations of meropenem detected in PBMCs lysate were below 30 ng/ml for the endolymphatic route of administration, and no clear signal was obtained for the intravenous route. This shows a higher content of meropenem in PBMC with the endolymphatic route of administration than with the intravenous route. In vitro experiments demonstrated elevated IL-1β secretion in infected macrophages after incubation with meropenem concentrations above 5 µg/ml, indicating activation of host innate immune response by pathogen-associated molecular patterns as a result of the release of damage-associated molecular patterns [20]. Apparently, an increase in the concentration of meropenem in the site of inflammation will lead to a more active death of bacterial cells, which in turn will stimulate the immune response more strongly.
Conclusion
Разработанные методики количественного определения меропенема в биологических образцах тяжелобольных пациентов валидированы в соответствии с действующими нормативными документами в области биоаналитических исследований и апробированы на реальных образцах, полученных от тяжелобольных пациентов. В дальнейшем данные методики планируется использовать как для дальнейшего изучения сравнительной фармакокинетики меропенема при различных путях введения, так и для терапевтического мониторинга концентрации меропенема на фоне антибактериальной терапии методом ВЭЖХ-ДМД.
Рассчитанные фармакокинетические параметры, полученные методом ВЭЖХ-ДМД, демонстрируют преимущества
эндолимфатического пути введения по сравнению с внутривенным на фармакокинетическом уровне. Однако для получения более достоверных выводов необходимо провести анализ на большей выборке пациентов, увеличив период исследования для наблюдения равновесных концентраций
в течение нескольких дней, а затем сравнить группы с внутривенным и эндолимфатическим путями введения для получения статистически значимо различающихся результатов. Также целесообразно продолжить изучение меропенема в мононуклеарных клетках периферической крови (МКПК) с использованием разработанного и валидированного метода ВЭЖХ-ЭСИ-МС/МС для лучшего понимания фармакокинетических особенностей эндолимфатического пути введения и причин их возникновения.
1. Внебольничная пневмония у взрослых: клинические рекомендации. Минздрав России. 2024. Дата обращения: 22.08.2025. https://cr.minzdrav.gov.ru/preview-cr/654_2
2. Инфекции мочевыводящих путей: клинические рекомендации. Минздрав России. 2024. Дата обращения: 22.08.2025. https://cr.minzdrav.gov.ru/view-cr/281_3
3. Менингококковая инфекция у детей: клинические рекомендации. Минздрав России. 2023. Дата обращения: 22.08.2025. https://cr.minzdrav.gov.ru/view-cr/58_2
References
1. Drusano G. Meropenem: laboratory and clinical data. Clin Microbiol Infect. 1997;3 Suppl 4:S51–S59. doi:10.1016/S1198-743X(14)65034-5
2. Salmon-Rousseau A, Martins C, Blot M, et al. Comparative review of imipenem/ cilastatin versus meropenem. Med Mal Infect. 2020;50(4):316–322. doi:10.1016/j.medmal.2020.01.001
3. Mouton JW, Touzw DJ, Horrevorts AM, Vinks AA. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin Pharmacokinet. 2000;39(3):185–201. doi:10.2165/00003088-200039030-00002.
4. Steffens NA, Zimmermann ES, Nichelle SM, Brucker N. Meropenem use and therapeutic drug monitoring in clinical practice: a literature review. J Clin Pharm Ther. 2021;46(3):610–621. doi:10.1111/jcpt.13369
5. Alsultan A, Dasuqi SA, Aljamaan F, et al. Pharmacokinetics of meropenem in critically ill patients in Saudi Arabia. Saudi Pharm J. 2021;29(11):1272-1277. doi:10.1016/j.jsps.2021.09.017.
6. Vtorenko VI, Esipov AV, Musailov VA, Shishlo VK. Lymphatic therapy in surgical practice. Surgical practice (Russia). 2014; 2014(3):29–34. (In Russian).
7. Petrenko NA, Groshilin VS, Davidenko AV, Lukash YV. Clinical efficiency of a comprehensive multifactorial approach to the treatment of forearm phlegmon. Ulyanovsk Medical and Biological Journal. 2017;2017(2):104–110. (In Russian). doi:10.23648/UMBJ.2017.26.6224
8. Vyrenkov YE, Kataev SI, Kharitonov VV, et al. Endolymphatic administration of drugs in the treatment of purulent-inflammatory diseases. Bulletin of the Ivanovo Medical Academy. 2015;20(4):57–63. (In Russian).
9. Syomkin VA, Nadtochiy AG, Vozgoment OV, Ivanova AA. Lymphatic therapy and its importance in the complex treatment of patients. Stomatologiia (Mosk). 2020;99(5):116–121. (In Russian). doi:10.17116/stomat202099051116
10. Roberts JA, Croom K, Adomakoh N. Continuous infusion of beta-lactam antibiotics: narrative review of systematic reviews, and implications for outpatient parenteral antibiotic therapy. Expert Rev Anti Infect Ther. 2023;21(4):375–385. doi:10.1080/14787210.2023.2184347
11. Razzazzadeh S, Darazam IA, Hajiesmaeili M, et al. Investigation of pharmacokinetic and clinical outcomes of various meropenem regimens in patients with ventilatorassociated pneumonia and augmented renal clearance. Eur J Clin Pharmacol. 2022;78(5):823–829. doi:10.1007/s00228-022-03291-5
12. Murray F, Yoo O, Brophy-Williams S, et al. Safety, tolerability and pharmacokinetics of subcutaneous meropenem as an alternative to intravenous administration. J Antimicrob Chemother. 2025;80(1):209–215. doi:10.1093/jac/dkae398
13. Wang Y, Li Z, Chen W, et al. Pharmacokinetics of meropenem in children with sepsis undergoing extracorporeal life support: A prospective observational study. J Clin Pharm Ther. 2021;46(3):754–761. doi:10.1111/jcpt.13344
14. Gan Y, Meng X, Lei N, Yu H, Zeng Q, Huang Q. Meropenem Pharmacokinetics and Target Attainment in Critically Ill Patients. Infect Drug Resist. 2023;16:3989– 3997. doi:10.2147/IDR.S408572
15. Corcione S, D’Avolio A, Loia RC, et al. Pharmacokinetics of meropenem in burn patients with infections caused by Gram-negative bacteria: Are we getting close to the right treatment? J Glob Antimicrob Resist. 2020;20:22–27. doi:10.1016/j.jgar.2019.06.011
16. Corona A, De Santis V, Agarossi A, et al. Antibiotic Therapy Strategies for Treating Gram-Negative Severe Infections in the Critically Ill: A Narrative Review. Antibiotics (Basel). 2023;12(8):1262. Published 2023 Jul 31. doi:10.3390/antibiotics12081262.
17. Raza A, Ngieng SC, Sime FB, et al. Oral meropenem for superbugs: challenges and opportunities. Drug Discov Today. 2021;26(2):551–560. doi:10.1016/j.drudis.2020.11.004
18. Selig DJ, Akers KS, Chung KK, Pruskowski KA, Livezey JR, Por ED. Meropenem pharmacokinetics in critically ill patients with or without burn treated with or without continuous veno-venous haemofiltration. Br J Clin Pharmacol. 2022;88(5):2156–2168. doi:10.1111/bcp.15138
19. Wise R, Logan M, Cooper M, Ashby JP, Andrews JM. Meropenem pharmacokinetics and penetration into an inflammatory exudate. Antimicrob Agents Chemother. 1990;34(8):1515–1517. doi:10.1128/AAC.34.8.1515
20. Olivença F, Pires D, Silveiro C, et al. Ethambutol and meropenem/clavulanate synergy promotes enhanced extracellular and intracellular killing of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2024;68(4):e0158623. doi:10.1128/aac.01586-23
About the Authors
V. N. TurenkoRussian Federation
Владислав Николаевич Туренко
96, корп. 119571, г. Москва, проспект Вернадского, д. 1
G. V. Ramenskaya
Russian Federation
Галина Владимировна Раменская
96, корп. 119571, г. Москва, проспект Вернадского, д. 1
Z. К. Karimova
Russian Federation
Зухранон К. Каримова
96, корп. 119571, г. Москва, проспект Вернадского, д. 1
А. V. Esipov
Russian Federation
Александр В. Есипов
143420, Московская область, Красногорский район, поселок Новый, д. 1
А. I. Pavlov
Russian Federation
Александр Иванович Павлов
143420, Московская область, Красногорский район, поселок Новый, д. 1
А. V. Filippov
Russian Federation
Александр Васильевич Филиппов
143420, Московская область, Красногорский район, поселок Новый, д. 1
А. М. Kislenko
Russian Federation
Андрей М. Кисленко
143420, Московская область, Красногорский район, поселок Новый, д. 1
V. V. Kharitonov
Russian Federation
Виталий Васильевич Харитонов
143420, Московская область, Красногорский район, поселок Новый, д. 1
E. Yusuf
Indonesia
Эдди Юсуф
Джл. Бульвар Рая № 2, Тиртаджая, Сукмаджая, Кота Депок, 16412
S. S. Korobov
Russian Federation
Степан С. Коробов
96, корп. 119571, г. Москва, проспект Вернадского, д. 1
S. N. Оrekhov
Russian Federation
Сергей Николаевич Орехов
проспект Вернадского, 96, корп. 119571, Москва, 1
V. V. Smirnov
Russian Federation
Валерий Васильевич Смирнов
96, корп. 119571, г. Москва, проспект Вернадского, д. 1
Review
For citations:
Turenko V.N., Ramenskaya G.V., Karimova Z.К., Esipov А.V., Pavlov А.I., Filippov А.V., Kislenko А.М., Kharitonov V.V., Yusuf E., Korobov S.S., Оrekhov S.N., Smirnov V.V. Development of approaches for evaluating the pharmacokinetics of meropenem during endolymphatic antibiotic treatment in critically ill patients. The BRICS Health Journal. 2025;2(2):24-35. https://doi.org/10.47093/3034-4700.2025.2.2.24-35








